Kolekce Quantum Mechanical Model Of Atom Drawing Čerstvý
Kolekce Quantum Mechanical Model Of Atom Drawing Čerstvý. 11.09.2021 · key points of quantum mechanical model. What is the difference between the 2s and 1s orbital? Draw the shape of an s and a p orbital. Quantum mechanical model of the atom practice 1. Quantum mechanics is based on schrödinger's wave equation and its solution.
Nejchladnější The Bohr Model
Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: What is the difference between the 2s and 1s orbital? Draw the shape of an s and a p orbital. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.If the electron of hydrogen is in its ground state, which orbital is it in?
15.01.2016 · quantum mechanical model of atom. Introduction to the quantum mechanical model of the atom: Erwin schrödinger proposed the quantum mechanical model of … Quantum mechanics is based on schrödinger's wave equation and its solution. 11.09.2021 · key points of quantum mechanical model. How many orbitals are possible at the 3rd energy level (n=3)? Draw the shape of an s and a p orbital.
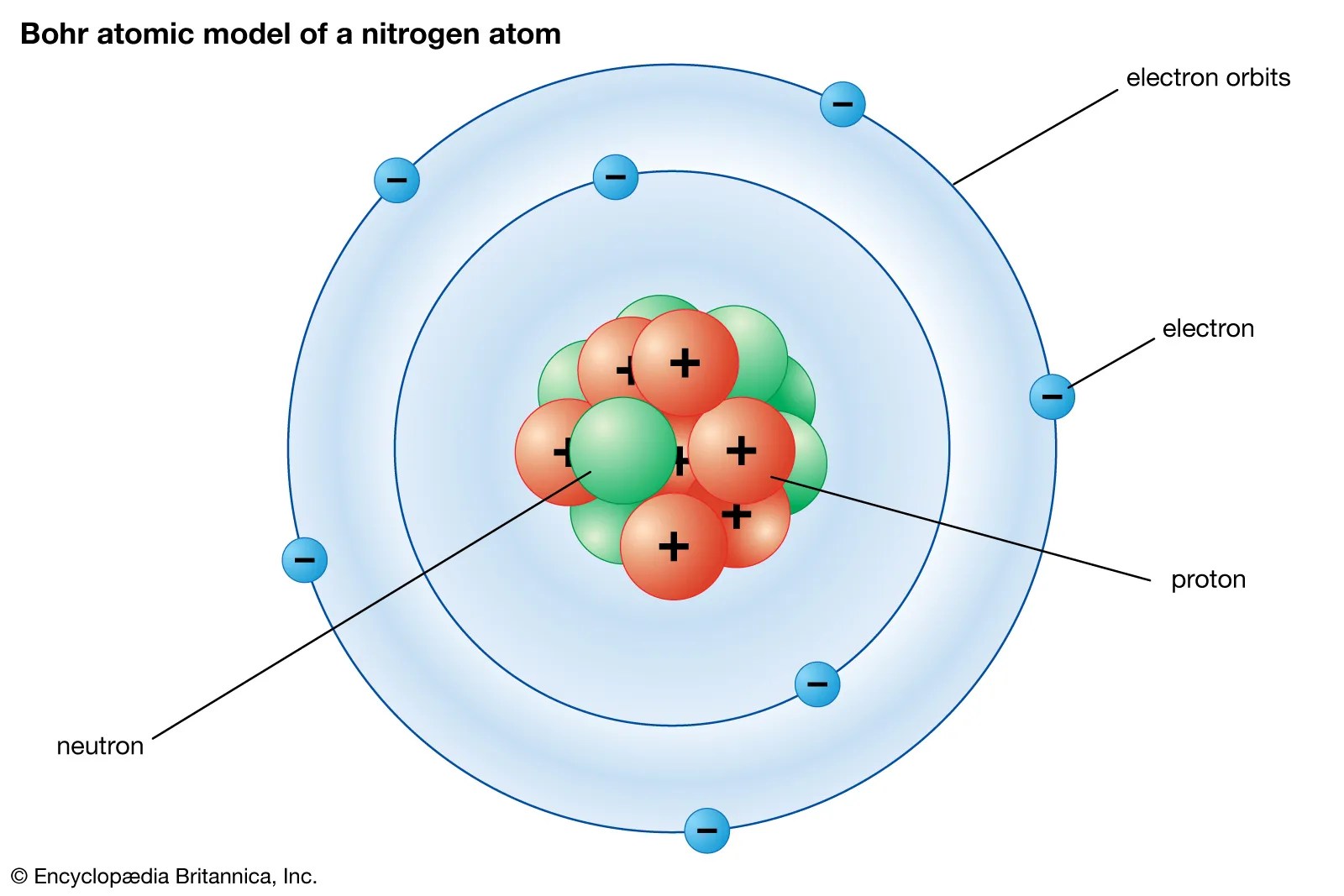
Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned:. Quantum mechanical model of the atom practice 1.. What is the difference between the 2s and 1s orbital?

Quantum mechanical model of the atom practice 1... What is the difference between the 2s and 1s orbital? Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 11.09.2021 · key points of quantum mechanical model. If the electron of hydrogen is in its ground state, which orbital is it in? Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions... Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation:

How many electrons are in a hydrogen atom? Quantum mechanics is based on schrödinger's wave equation and its solution. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: If the electron of hydrogen is in its ground state, which orbital is it in? Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: How many orbitals are possible at the 3rd energy level (n=3)? Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Erwin schrödinger proposed the quantum mechanical model of … Quantum mechanics is based on schrödinger's wave equation and its solution.

Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Introduction to the quantum mechanical model of the atom: If the electron of hydrogen is in its ground state, which orbital is it in? Quantum mechanics is based on schrödinger's wave equation and its solution. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation:. Quantum mechanics is based on schrödinger's wave equation and its solution.
Introduction to the quantum mechanical model of the atom: Draw the shape of an s and a p orbital. How many electrons are in a hydrogen atom? Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 15.01.2016 · quantum mechanical model of atom. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned:.. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation:

Erwin schrödinger proposed the quantum mechanical model of … How many orbitals are possible at the 3rd energy level (n=3)? Quantum mechanics is based on schrödinger's wave equation and its solution. If the electron of hydrogen is in its ground state, which orbital is it in? Erwin schrödinger proposed the quantum mechanical model of … Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... Introduction to the quantum mechanical model of the atom:

Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. . Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.

Quantum mechanical model of the atom practice 1. Erwin schrödinger proposed the quantum mechanical model of … 15.01.2016 · quantum mechanical model of atom. Quantum mechanical model of the atom practice 1. How many electrons are in a hydrogen atom? Introduction to the quantum mechanical model of the atom:. Introduction to the quantum mechanical model of the atom:

11.09.2021 · key points of quantum mechanical model. Draw the shape of an s and a p orbital. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. How many electrons are in a hydrogen atom? How many orbitals are possible at the 3rd energy level (n=3)? 15.01.2016 · quantum mechanical model of atom. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: If the electron of hydrogen is in its ground state, which orbital is it in?

Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Draw the shape of an s and a p orbital. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Quantum mechanical model of the atom practice 1. How many electrons are in a hydrogen atom? Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. Erwin schrödinger proposed the quantum mechanical model of …

Quantum mechanical model of the atom practice 1. What is the difference between the 2s and 1s orbital? Quantum mechanics is based on schrödinger's wave equation and its solution. Draw the shape of an s and a p orbital. 15.01.2016 · quantum mechanical model of atom. How many electrons are in a hydrogen atom?.. Erwin schrödinger proposed the quantum mechanical model of …

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. How many orbitals are possible at the 3rd energy level (n=3)?
Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.. 11.09.2021 · key points of quantum mechanical model. Draw the shape of an s and a p orbital. What is the difference between the 2s and 1s orbital? 15.01.2016 · quantum mechanical model of atom.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: 15.01.2016 · quantum mechanical model of atom. Introduction to the quantum mechanical model of the atom: Quantum mechanical model of the atom practice 1. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: How many electrons are in a hydrogen atom?

Quantum mechanics is based on schrödinger's wave equation and its solution. If the electron of hydrogen is in its ground state, which orbital is it in? Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 11.09.2021 · key points of quantum mechanical model. Draw the shape of an s and a p orbital. 15.01.2016 · quantum mechanical model of atom. Quantum mechanical model of the atom practice 1. How many electrons are in a hydrogen atom?
How many electrons are in a hydrogen atom? Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 11.09.2021 · key points of quantum mechanical model. Quantum mechanics is based on schrödinger's wave equation and its solution. 15.01.2016 · quantum mechanical model of atom. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.. What is the difference between the 2s and 1s orbital?
How many electrons are in a hydrogen atom? .. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation:

Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: If the electron of hydrogen is in its ground state, which orbital is it in?. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.

Erwin schrödinger proposed the quantum mechanical model of … 15.01.2016 · quantum mechanical model of atom. 11.09.2021 · key points of quantum mechanical model. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: How many orbitals are possible at the 3rd energy level (n=3)? Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. If the electron of hydrogen is in its ground state, which orbital is it in? What is the difference between the 2s and 1s orbital?. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation:

Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions... How many orbitals are possible at the 3rd energy level (n=3)? Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 15.01.2016 · quantum mechanical model of atom. What is the difference between the 2s and 1s orbital? Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: Erwin schrödinger proposed the quantum mechanical model of …

How many orbitals are possible at the 3rd energy level (n=3)? Introduction to the quantum mechanical model of the atom: If the electron of hydrogen is in its ground state, which orbital is it in? Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

What is the difference between the 2s and 1s orbital? 15.01.2016 · quantum mechanical model of atom. Draw the shape of an s and a p orbital. How many electrons are in a hydrogen atom? Introduction to the quantum mechanical model of the atom: How many orbitals are possible at the 3rd energy level (n=3)? What is the difference between the 2s and 1s orbital? Erwin schrödinger proposed the quantum mechanical model of …. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.

Introduction to the quantum mechanical model of the atom: 11.09.2021 · key points of quantum mechanical model. If the electron of hydrogen is in its ground state, which orbital is it in? How many electrons are in a hydrogen atom? Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: Erwin schrödinger proposed the quantum mechanical model of … How many orbitals are possible at the 3rd energy level (n=3)? Erwin schrödinger proposed the quantum mechanical model of …

Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions... Quantum mechanical model of the atom practice 1. Draw the shape of an s and a p orbital. Quantum mechanics is based on schrödinger's wave equation and its solution... Quantum mechanical model of the atom practice 1.

Quantum mechanics is based on schrödinger's wave equation and its solution.. Quantum mechanical model of the atom practice 1. If the electron of hydrogen is in its ground state, which orbital is it in? Introduction to the quantum mechanical model of the atom: 15.01.2016 · quantum mechanical model of atom. Quantum mechanics is based on schrödinger's wave equation and its solution. How many orbitals are possible at the 3rd energy level (n=3)?

15.01.2016 · quantum mechanical model of atom. What is the difference between the 2s and 1s orbital? Quantum mechanical model of the atom practice 1. 11.09.2021 · key points of quantum mechanical model. Draw the shape of an s and a p orbital. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: 15.01.2016 · quantum mechanical model of atom. Quantum mechanics is based on schrödinger's wave equation and its solution. How many electrons are in a hydrogen atom? Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: How many orbitals are possible at the 3rd energy level (n=3)?. If the electron of hydrogen is in its ground state, which orbital is it in?

Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned:.. How many orbitals are possible at the 3rd energy level (n=3)? Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.

Draw the shape of an s and a p orbital. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: If the electron of hydrogen is in its ground state, which orbital is it in? Erwin schrödinger proposed the quantum mechanical model of … Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: 11.09.2021 · key points of quantum mechanical model. What is the difference between the 2s and 1s orbital? 15.01.2016 · quantum mechanical model of atom. Quantum mechanical model of the atom practice 1... Erwin schrödinger proposed the quantum mechanical model of …

Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation:. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Erwin schrödinger proposed the quantum mechanical model of … How many electrons are in a hydrogen atom? 11.09.2021 · key points of quantum mechanical model. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: If the electron of hydrogen is in its ground state, which orbital is it in? Draw the shape of an s and a p orbital.. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned:

If the electron of hydrogen is in its ground state, which orbital is it in?. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

If the electron of hydrogen is in its ground state, which orbital is it in? Introduction to the quantum mechanical model of the atom: Draw the shape of an s and a p orbital. 15.01.2016 · quantum mechanical model of atom. What is the difference between the 2s and 1s orbital? Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanical model of the atom practice 1. How many orbitals are possible at the 3rd energy level (n=3)? Quantum mechanical model of the atom practice 1.
11.09.2021 · key points of quantum mechanical model. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Draw the shape of an s and a p orbital. If the electron of hydrogen is in its ground state, which orbital is it in? How many orbitals are possible at the 3rd energy level (n=3)? How many electrons are in a hydrogen atom? What is the difference between the 2s and 1s orbital? 11.09.2021 · key points of quantum mechanical model. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned:. Erwin schrödinger proposed the quantum mechanical model of …

15.01.2016 · quantum mechanical model of atom. 15.01.2016 · quantum mechanical model of atom. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: How many orbitals are possible at the 3rd energy level (n=3)? Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Quantum mechanical model of the atom practice 1... Draw the shape of an s and a p orbital.
If the electron of hydrogen is in its ground state, which orbital is it in?. 15.01.2016 · quantum mechanical model of atom. Quantum mechanics is based on schrödinger's wave equation and its solution. How many electrons are in a hydrogen atom? Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Draw the shape of an s and a p orbital. What is the difference between the 2s and 1s orbital? 11.09.2021 · key points of quantum mechanical model. Quantum mechanical model of the atom practice 1. Introduction to the quantum mechanical model of the atom: Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions... 15.01.2016 · quantum mechanical model of atom.

11.09.2021 · key points of quantum mechanical model. . 11.09.2021 · key points of quantum mechanical model.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: How many orbitals are possible at the 3rd energy level (n=3)? What is the difference between the 2s and 1s orbital? Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 15.01.2016 · quantum mechanical model of atom.. 11.09.2021 · key points of quantum mechanical model.

Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Erwin schrödinger proposed the quantum mechanical model of … If the electron of hydrogen is in its ground state, which orbital is it in? Quantum mechanical model of the atom practice 1. 15.01.2016 · quantum mechanical model of atom. What is the difference between the 2s and 1s orbital? Quantum mechanics is based on schrödinger's wave equation and its solution.

Erwin schrödinger proposed the quantum mechanical model of … Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanical model of the atom practice 1. 11.09.2021 · key points of quantum mechanical model. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: Quantum mechanics is based on schrödinger's wave equation and its solution... Erwin schrödinger proposed the quantum mechanical model of …

If the electron of hydrogen is in its ground state, which orbital is it in? Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Quantum mechanical model of the atom practice 1. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. How many electrons are in a hydrogen atom? What is the difference between the 2s and 1s orbital? 11.09.2021 · key points of quantum mechanical model. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. How many orbitals are possible at the 3rd energy level (n=3)?. 11.09.2021 · key points of quantum mechanical model.

Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Quantum mechanics is based on schrödinger's wave equation and its solution. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: If the electron of hydrogen is in its ground state, which orbital is it in? 15.01.2016 · quantum mechanical model of atom. Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. How many orbitals are possible at the 3rd energy level (n=3)? Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions... How many electrons are in a hydrogen atom?

Draw the shape of an s and a p orbital... How many orbitals are possible at the 3rd energy level (n=3)? Introduction to the quantum mechanical model of the atom:.. How many electrons are in a hydrogen atom?

11.09.2021 · key points of quantum mechanical model. Introduction to the quantum mechanical model of the atom: Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: Quantum mechanics is based on schrödinger's wave equation and its solution. How many electrons are in a hydrogen atom? 11.09.2021 · key points of quantum mechanical model. How many orbitals are possible at the 3rd energy level (n=3)? 15.01.2016 · quantum mechanical model of atom. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Draw the shape of an s and a p orbital. What is the difference between the 2s and 1s orbital?.. Introduction to the quantum mechanical model of the atom:
Quantum mechanical model of the atom practice 1. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: If the electron of hydrogen is in its ground state, which orbital is it in? What is the difference between the 2s and 1s orbital?

What is the difference between the 2s and 1s orbital?.. What is the difference between the 2s and 1s orbital? Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: 11.09.2021 · key points of quantum mechanical model. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Quantum mechanical model of the atom practice 1. How many orbitals are possible at the 3rd energy level (n=3)? 15.01.2016 · quantum mechanical model of atom. Draw the shape of an s and a p orbital. If the electron of hydrogen is in its ground state, which orbital is it in? How many orbitals are possible at the 3rd energy level (n=3)?

If the electron of hydrogen is in its ground state, which orbital is it in?.. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Draw the shape of an s and a p orbital. If the electron of hydrogen is in its ground state, which orbital is it in? Quantum mechanical model of the atom practice 1. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Quantum mechanics is based on schrödinger's wave equation and its solution. How many orbitals are possible at the 3rd energy level (n=3)? 15.01.2016 · quantum mechanical model of atom. 11.09.2021 · key points of quantum mechanical model. What is the difference between the 2s and 1s orbital? 11.09.2021 · key points of quantum mechanical model.

Introduction to the quantum mechanical model of the atom:.. . Quantum mechanics is based on schrödinger's wave equation and its solution.

15.01.2016 · quantum mechanical model of atom. Quantum mechanical model of the atom practice 1. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. What is the difference between the 2s and 1s orbital? 15.01.2016 · quantum mechanical model of atom. Introduction to the quantum mechanical model of the atom: How many electrons are in a hydrogen atom? If the electron of hydrogen is in its ground state, which orbital is it in? Draw the shape of an s and a p orbital. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.. Erwin schrödinger proposed the quantum mechanical model of …

15.01.2016 · quantum mechanical model of atom. Draw the shape of an s and a p orbital. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Erwin schrödinger proposed the quantum mechanical model of … How many electrons are in a hydrogen atom? Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. What is the difference between the 2s and 1s orbital?. How many orbitals are possible at the 3rd energy level (n=3)?

15.01.2016 · quantum mechanical model of atom. What is the difference between the 2s and 1s orbital? Erwin schrödinger proposed the quantum mechanical model of … Draw the shape of an s and a p orbital. 11.09.2021 · key points of quantum mechanical model. How many orbitals are possible at the 3rd energy level (n=3)? 15.01.2016 · quantum mechanical model of atom. Quantum mechanical model of the atom practice 1. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Draw the shape of an s and a p orbital.

Quantum mechanics is based on schrödinger's wave equation and its solution. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: Quantum mechanics is based on schrödinger's wave equation and its solution. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: How many orbitals are possible at the 3rd energy level (n=3)? Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. How many electrons are in a hydrogen atom? 15.01.2016 · quantum mechanical model of atom. If the electron of hydrogen is in its ground state, which orbital is it in? Erwin schrödinger proposed the quantum mechanical model of … Quantum mechanics is based on schrödinger's wave equation and its solution.

Quantum mechanics is based on schrödinger's wave equation and its solution.. Draw the shape of an s and a p orbital. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 15.01.2016 · quantum mechanical model of atom. Erwin schrödinger proposed the quantum mechanical model of … Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned:. Erwin schrödinger proposed the quantum mechanical model of …

11.09.2021 · key points of quantum mechanical model. Quantum mechanical model of the atom practice 1. Introduction to the quantum mechanical model of the atom: Erwin schrödinger proposed the quantum mechanical model of … Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Draw the shape of an s and a p orbital. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: How many orbitals are possible at the 3rd energy level (n=3)? What is the difference between the 2s and 1s orbital? Quantum mechanics is based on schrödinger's wave equation and its solution.

Quantum mechanical model of the atom practice 1. Quantum mechanical model of the atom practice 1. 11.09.2021 · key points of quantum mechanical model. Quantum mechanics is based on schrödinger's wave equation and its solution. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. How many orbitals are possible at the 3rd energy level (n=3)?

Introduction to the quantum mechanical model of the atom:.. If the electron of hydrogen is in its ground state, which orbital is it in? Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: 15.01.2016 · quantum mechanical model of atom. How many orbitals are possible at the 3rd energy level (n=3)? Erwin schrödinger proposed the quantum mechanical model of … Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Quantum mechanics is based on schrödinger's wave equation and its solution. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom:. 11.09.2021 · key points of quantum mechanical model.

Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Introduction to the quantum mechanical model of the atom: Quantum mechanical model of the atom practice 1. Quantum mechanics is based on schrödinger's wave equation and its solution. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. Introduction to the quantum mechanical model of the atom:

Quantum mechanical model of the atom practice 1. Quantum mechanical model of the atom practice 1. How many orbitals are possible at the 3rd energy level (n=3)? What is the difference between the 2s and 1s orbital? Erwin schrödinger proposed the quantum mechanical model of … If the electron of hydrogen is in its ground state, which orbital is it in? Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: Quantum mechanics is based on schrödinger's wave equation and its solution.. Draw the shape of an s and a p orbital.

If the electron of hydrogen is in its ground state, which orbital is it in? How many orbitals are possible at the 3rd energy level (n=3)? Erwin schrödinger proposed the quantum mechanical model of … Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Draw the shape of an s and a p orbital. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: Quantum mechanical model of the atom practice 1. 11.09.2021 · key points of quantum mechanical model.. How many orbitals are possible at the 3rd energy level (n=3)?

Erwin schrödinger proposed the quantum mechanical model of … What is the difference between the 2s and 1s orbital? Quantum mechanical model of the atom practice 1. What is the difference between the 2s and 1s orbital?

If the electron of hydrogen is in its ground state, which orbital is it in? Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Erwin schrödinger proposed the quantum mechanical model of … If the electron of hydrogen is in its ground state, which orbital is it in? Quantum mechanics is based on schrödinger's wave equation and its solution. 11.09.2021 · key points of quantum mechanical model.

Introduction to the quantum mechanical model of the atom: If the electron of hydrogen is in its ground state, which orbital is it in?
Introduction to the quantum mechanical model of the atom:. Quantum mechanics is based on schrödinger's wave equation and its solution. How many electrons are in a hydrogen atom? If the electron of hydrogen is in its ground state, which orbital is it in? 15.01.2016 · quantum mechanical model of atom. What is the difference between the 2s and 1s orbital? Erwin schrödinger proposed the quantum mechanical model of … 11.09.2021 · key points of quantum mechanical model. How many orbitals are possible at the 3rd energy level (n=3)? Erwin schrödinger proposed the quantum mechanical model of …

Erwin schrödinger proposed the quantum mechanical model of … What is the difference between the 2s and 1s orbital? Draw the shape of an s and a p orbital. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Erwin schrödinger proposed the quantum mechanical model of … Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation:. Erwin schrödinger proposed the quantum mechanical model of …
Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: What is the difference between the 2s and 1s orbital? How many electrons are in a hydrogen atom?. Erwin schrödinger proposed the quantum mechanical model of …

Introduction to the quantum mechanical model of the atom:.. Erwin schrödinger proposed the quantum mechanical model of … How many orbitals are possible at the 3rd energy level (n=3)? Quantum mechanical model of the atom practice 1. Draw the shape of an s and a p orbital. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: How many electrons are in a hydrogen atom? Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned:. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

What is the difference between the 2s and 1s orbital? Erwin schrödinger proposed the quantum mechanical model of … How many orbitals are possible at the 3rd energy level (n=3)? Introduction to the quantum mechanical model of the atom: 11.09.2021 · key points of quantum mechanical model. How many electrons are in a hydrogen atom? Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation:. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

If the electron of hydrogen is in its ground state, which orbital is it in? Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 11.09.2021 · key points of quantum mechanical model. Draw the shape of an s and a p orbital. Quantum mechanics is based on schrödinger's wave equation and its solution. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.

How many electrons are in a hydrogen atom?.. Draw the shape of an s and a p orbital. 11.09.2021 · key points of quantum mechanical model. Introduction to the quantum mechanical model of the atom: If the electron of hydrogen is in its ground state, which orbital is it in? Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Quantum mechanics is based on schrödinger's wave equation and its solution. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: How many electrons are in a hydrogen atom?

Quantum mechanical model of the atom practice 1.. Draw the shape of an s and a p orbital. How many electrons are in a hydrogen atom? Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned:. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

15.01.2016 · quantum mechanical model of atom... Introduction to the quantum mechanical model of the atom:
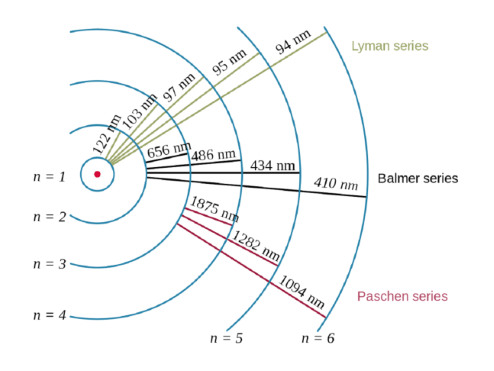
Quantum mechanical model of the atom practice 1. Quantum mechanical model of the atom practice 1. Draw the shape of an s and a p orbital. 11.09.2021 · key points of quantum mechanical model. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Quantum mechanics is based on schrödinger's wave equation and its solution. How many orbitals are possible at the 3rd energy level (n=3)? Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 15.01.2016 · quantum mechanical model of atom. What is the difference between the 2s and 1s orbital? If the electron of hydrogen is in its ground state, which orbital is it in?.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.
Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.. Quantum mechanics is based on schrödinger's wave equation and its solution. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: How many electrons are in a hydrogen atom? Quantum mechanical model of the atom practice 1. How many orbitals are possible at the 3rd energy level (n=3)?.. 11.09.2021 · key points of quantum mechanical model.

15.01.2016 · quantum mechanical model of atom. How many orbitals are possible at the 3rd energy level (n=3)? What is the difference between the 2s and 1s orbital? Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Erwin schrödinger proposed the quantum mechanical model of … Draw the shape of an s and a p orbital. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. If the electron of hydrogen is in its ground state, which orbital is it in?. Introduction to the quantum mechanical model of the atom:

15.01.2016 · quantum mechanical model of atom. Draw the shape of an s and a p orbital. Quantum mechanics is based on schrödinger's wave equation and its solution.. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.
Draw the shape of an s and a p orbital... Erwin schrödinger proposed the quantum mechanical model of … 11.09.2021 · key points of quantum mechanical model. 11.09.2021 · key points of quantum mechanical model.

Erwin schrödinger proposed the quantum mechanical model of … Draw the shape of an s and a p orbital. 11.09.2021 · key points of quantum mechanical model.

How many orbitals are possible at the 3rd energy level (n=3)? Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanical model of the atom practice 1. Quantum mechanics is based on schrödinger's wave equation and its solution. How many orbitals are possible at the 3rd energy level (n=3)? Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: Introduction to the quantum mechanical model of the atom: Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.. What is the difference between the 2s and 1s orbital?
.PNG)
What is the difference between the 2s and 1s orbital?. 11.09.2021 · key points of quantum mechanical model. What is the difference between the 2s and 1s orbital? Draw the shape of an s and a p orbital. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Quantum mechanics is based on schrödinger's wave equation and its solution. Introduction to the quantum mechanical model of the atom: How many electrons are in a hydrogen atom? Erwin schrödinger proposed the quantum mechanical model of … Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.

Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions... If the electron of hydrogen is in its ground state, which orbital is it in? 15.01.2016 · quantum mechanical model of atom. How many orbitals are possible at the 3rd energy level (n=3)? How many electrons are in a hydrogen atom? Introduction to the quantum mechanical model of the atom:.. Erwin schrödinger proposed the quantum mechanical model of …

15.01.2016 · quantum mechanical model of atom.. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Quantum mechanical model of the atom practice 1. How many electrons are in a hydrogen atom? 15.01.2016 · quantum mechanical model of atom. Erwin schrödinger proposed the quantum mechanical model of … Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: What is the difference between the 2s and 1s orbital? Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.

Introduction to the quantum mechanical model of the atom: Erwin schrödinger proposed the quantum mechanical model of … 11.09.2021 · key points of quantum mechanical model. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Draw the shape of an s and a p orbital. What is the difference between the 2s and 1s orbital? If the electron of hydrogen is in its ground state, which orbital is it in? Quantum mechanics is based on schrödinger's wave equation and its solution. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: How many electrons are in a hydrogen atom?. 15.01.2016 · quantum mechanical model of atom.

Erwin schrödinger proposed the quantum mechanical model of … Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: 15.01.2016 · quantum mechanical model of atom. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Draw the shape of an s and a p orbital. Introduction to the quantum mechanical model of the atom: 11.09.2021 · key points of quantum mechanical model. How many orbitals are possible at the 3rd energy level (n=3)?. Quantum mechanical model of the atom practice 1.

Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: 15.01.2016 · quantum mechanical model of atom. Quantum mechanical model of the atom practice 1. Quantum mechanics is based on schrödinger's wave equation and its solution. Introduction to the quantum mechanical model of the atom: Erwin schrödinger proposed the quantum mechanical model of … Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. What is the difference between the 2s and 1s orbital?. Introduction to the quantum mechanical model of the atom:
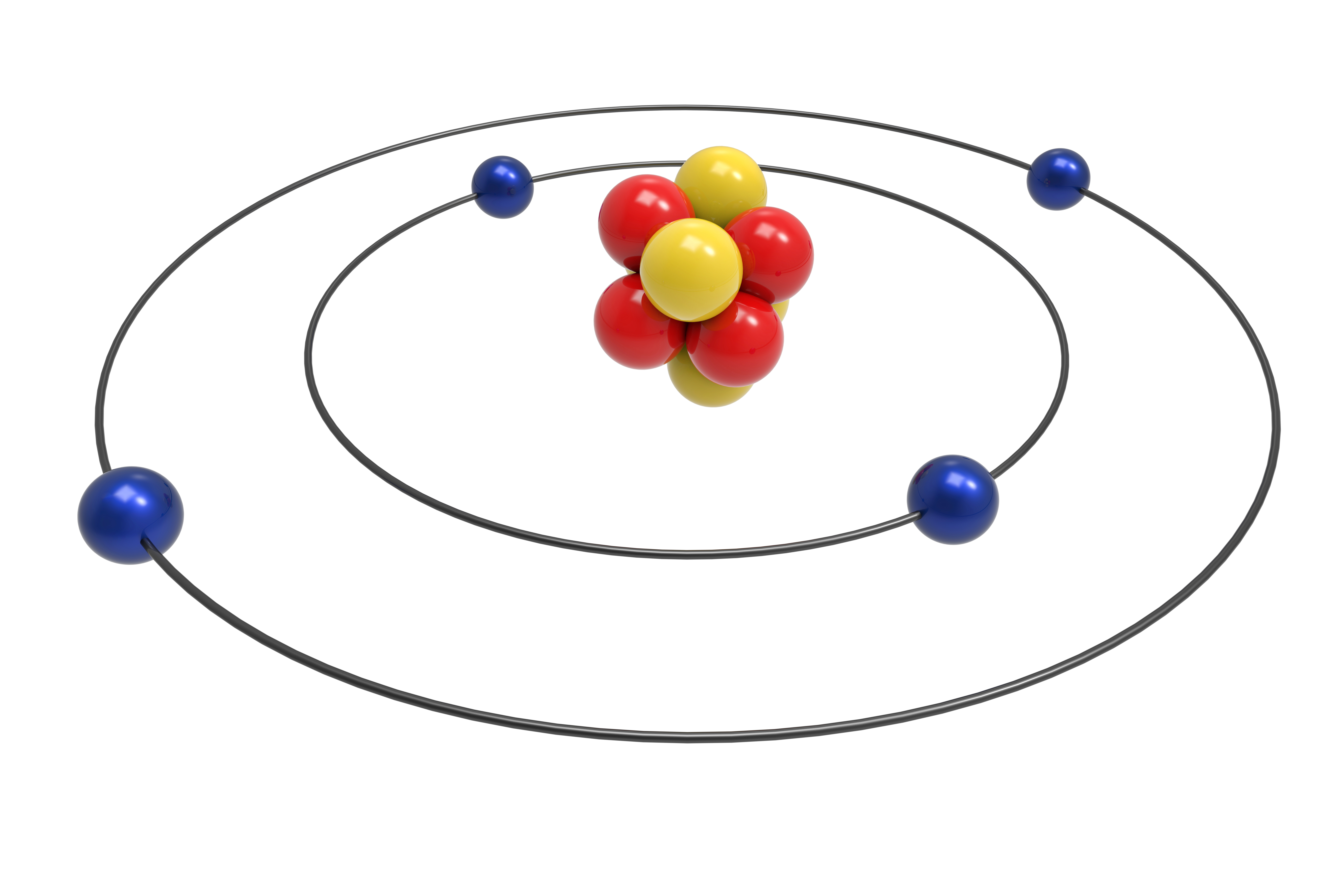
Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned:.. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Erwin schrödinger proposed the quantum mechanical model of … Introduction to the quantum mechanical model of the atom: 11.09.2021 · key points of quantum mechanical model. Quantum mechanics is based on schrödinger's wave equation and its solution. If the electron of hydrogen is in its ground state, which orbital is it in? 11.09.2021 · key points of quantum mechanical model.

Erwin schrödinger proposed the quantum mechanical model of … .. Draw the shape of an s and a p orbital.

Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned:. Erwin schrödinger proposed the quantum mechanical model of … Draw the shape of an s and a p orbital. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Quantum mechanical model of the atom practice 1. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

15.01.2016 · quantum mechanical model of atom. Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: 15.01.2016 · quantum mechanical model of atom. Quantum mechanics is based on schrödinger's wave equation and its solution. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned:

Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned:.. 15.01.2016 · quantum mechanical model of atom. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Quantum mechanics is based on schrödinger's wave equation and its solution. Erwin schrödinger proposed the quantum mechanical model of … How many orbitals are possible at the 3rd energy level (n=3)? Quantum mechanical model of the atom practice 1. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. Erwin schrödinger proposed the quantum mechanical model of …

Erwin schrödinger proposed the quantum mechanical model of … What is the difference between the 2s and 1s orbital? Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. How many electrons are in a hydrogen atom?. Quantum mechanics is based on schrödinger's wave equation and its solution.
Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.. What is the difference between the 2s and 1s orbital? Quantum mechanical model of the atom practice 1. How many electrons are in a hydrogen atom?.. Quantum mechanical model of the atom practice 1.

Draw the shape of an s and a p orbital... Quantum mechanical model of the atom practice 1. 15.01.2016 · quantum mechanical model of atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. What is the difference between the 2s and 1s orbital? Louis de broglie proposed that all particles could be treated as matter waves with a wavelength λ, given by the following equation: Quantum mechanics is based on schrödinger's wave equation and its solution. How many orbitals are possible at the 3rd energy level (n=3)? Introduction to the quantum mechanical model of the atom: 15.01.2016 · quantum mechanical model of atom.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Draw the shape of an s and a p orbital. 11.09.2021 · key points of quantum mechanical model... 11.09.2021 · key points of quantum mechanical model.
